6 Steps to Innovation:
How Scientists Make Safe, Effective Vaccines
Vaccines stimulate the immune system to help protect against dangerous infections. Doing this right takes a lot of research.
We explain the steps that make it possible.
Vaccines accomplish something big — they can help save lives and prevent disease, and they do it in a simple way: by teaching your body to protect itself against a particular germ, without giving you the illness.
Developing an effective, safe vaccine takes a great deal of work. At Novavax, scientists carefully design and test new vaccines intended to prevent illnesses.
Here's a guide to the crucial steps scientists must take to get a vaccine from the drawing board to the doctor’s office:
1. Identify The Target
First, researchers need to decide if it even makes sense to develop a vaccine against a certain disease. For example, the common cold probably wouldn’t be a good candidate because many different germs cause colds and the effects are usually relatively mild.
2. Pick A Strategy
While all vaccines are designed to trigger a response from your immune system, they can accomplish this in different ways. Scientists first research a virus and figure out what part of it to teach the immune system to recognize, then they need to introduce that part to the immune system. Novavax uses viral proteins, which are packaged into nanoparticles, to accomplish this. These nanoparticles help your immune system recognize the protein from different angles. Because there’s no actual virus, just protein, they can’t cause disease.

3. Start Testing
Next, scientists begin preliminary lab tests, such as testing a potential vaccine candidate with cell samples. If these results look promising, researchers often move on to tests with animals.
4. Launch Clinical Trials
If lab tests suggest that a potential vaccine candidate is safe and produces the immune response that’s needed, it can move on to human tests with people who volunteer to take part. These experiments are known as clinical trials, and they are broken into three parts.
- Phase 1: The first phase focuses on safety. A small number of people receive the potential vaccine candidate and scientists monitor these participants for side effects.
- Phase 2: This time, a larger group of people receive the potential vaccine candidate. Scientists work on determining an effective dosage and continue watching for any side effects.
- Phase 3: Even more people, from hundreds to thousands, participate during this phase. Their results — whether or not they contracted the disease or experienced side effects — are compared to those of people who received a treatment that has no effect, called a placebo, instead of the new vaccine.
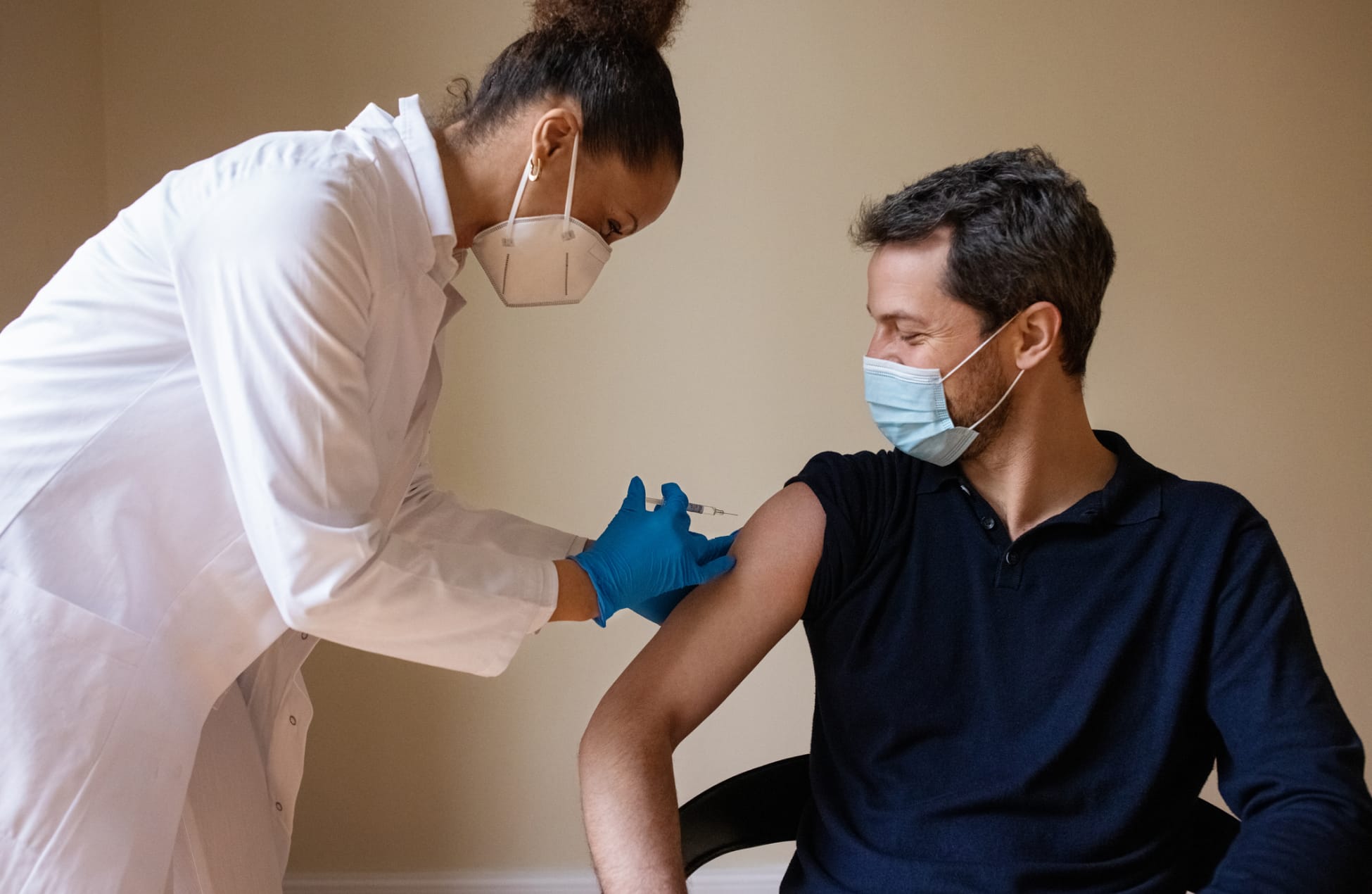
5. Getting The Green Light
The U.S. Food and Drug Administration (FDA) evaluates clinical trial data and other information. This extensive review helps determine whether the protection offered by the potential vaccine candidate outweighs any side effects. Only if a vaccine reaches this high standard does the FDA approve it.
6. Distribute The Vaccine
With the FDA’s approval, healthcare providers can begin giving the vaccine to patients. Even after the general public has begun receiving a vaccine, monitoring continues to make sure it is safe and effective.
At Novavax, scientists are working diligently at every stage in this process. Through hard work and care, they aim to efficiently deliver effective new vaccines while protecting the safety of the people who receive them.
-
 Twitter
Twitter
-
 Facebook
Facebook
-
 Pinterest
Pinterest